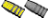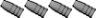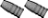[QUOTE=Larry Gibson;I finally got around to pulling the rest of the bullets and about 2/3 had severe corrosion inside the cases and on the bases of the bullets. Picture shows 2 of the fired cases including the blown primer. Middle case shows corrosion inside the cases. Bullet on right shows corrosion on the base. Other 2 bullets cleaned up nice with polishing. Powder looks good with no discoloration and smells good but will make good fertilizer anyway..........
Larry Gibson QUOTE]
Dam Larry
Now I'm worried. I have a number of GI spam cans of M1 ammo in M1 clips, that I've had for 20, 30 or even 40 yrs, sitting around waiting for the revolution.
I havn't opened one in years. I do have several 30 cal ammo cans full of lc surplus & NM that I use for standardization testing. I can't remember if they are the same.
Also have sealed in plastic in the wood cases 308 in Austrian, Argentine & British surplus at least 20yo.
Don't remember ever having a problem but I do remember some with external corrosion on the brass.
I'd hate to open airtight cans or sealed plastic to check.
What do you guys think?
Tom

|
   
   
|


|



 Reply With Quote
Reply With Quote